Crucial professions in vital processes
Explanation results Corona Covid-19 laboratory test for antibodies
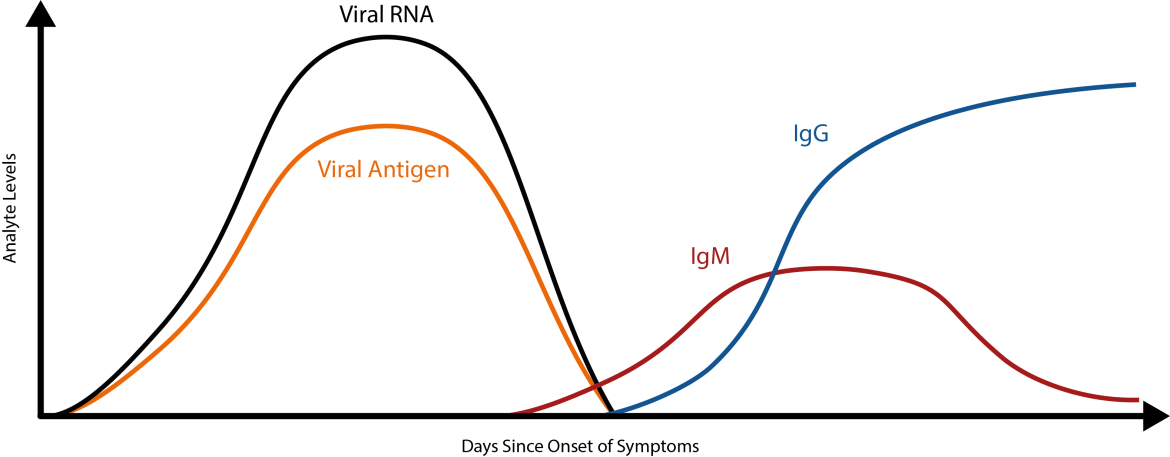
When are which covid-19 tests possible? When can you see which antibodies?
seroconversion covid-19 antibody test
EUROIMMUN Anti-SARS-CoV-2-ELISA
US RIVM approves EUROIMMUN IgG antibody test

G. van Lit
at 11 Mar 2021Marc Vandermeiren
at Jun 05, 2021angela
at Jun 30, 2021BSP Mate
at 27 Mar 2021wester
at Feb 28, 2021wester
at Feb 28, 2021